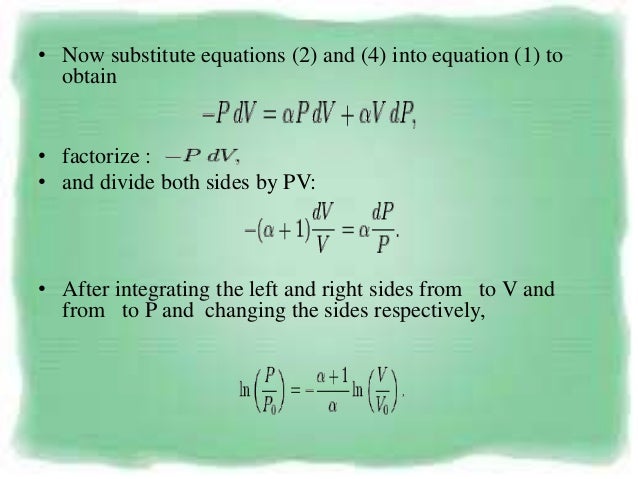
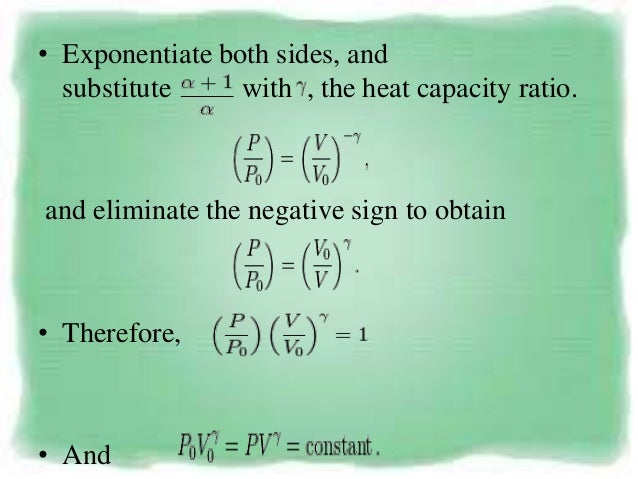
Pvt Relationship In Adiabatic Process

Chapter 3c The First Law Closed Systems Diesel Cycle Engines Updated 3 19 13

Co Efficient Of Volume Expansion Isothermal Compressibility And Adiabatic Compressibility Youtube

Adiabatic Process P V T Relation Youtube
How To Prove Math Pv Gamma Text Constant Math For An Adiabatic Process Quora
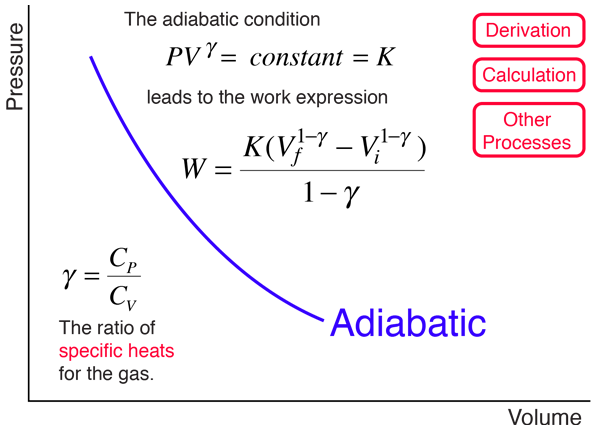
Adiabatic Processes

Thermal Engineering 10
(d) Now we can go back to the relationship we obtained for T dS (which we know is 0 since the process is adiabatic and reversible) T dS = 0 = CpdT − T V αdP dT αV = dP T Cp Tf αV ln = ΔP Ti Cp αV αM Tf = Ti exp ΔP = Ti exp ΔP Cp Cpρ 0.5kg ∗ 49.5× 10−6K−1 ∗ 5000atm ∗ 1.01× 105P a/atm Tf = 298exp.

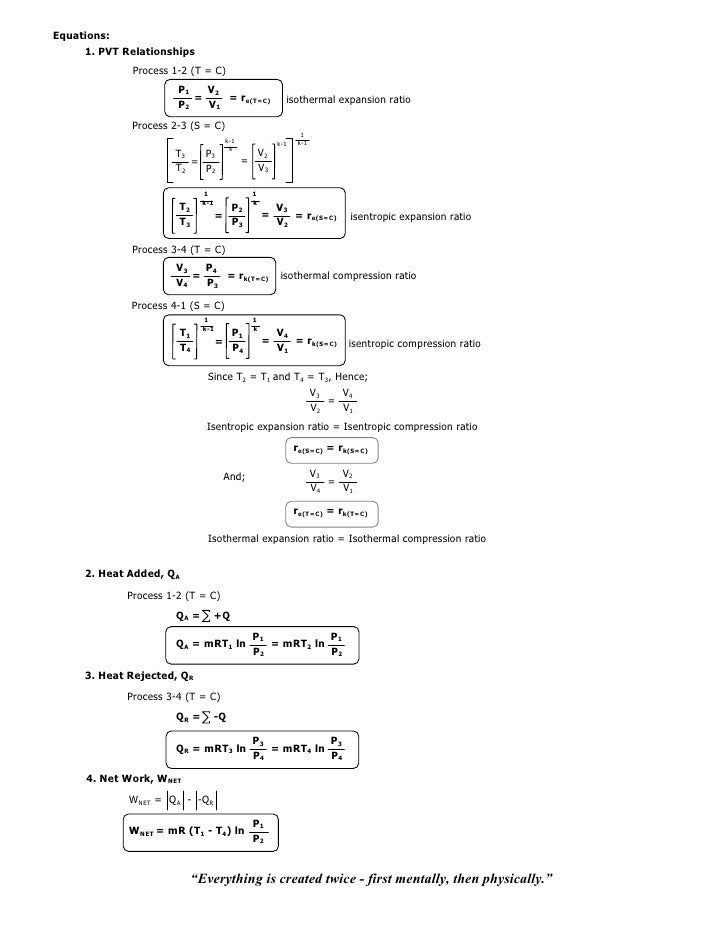
Pvt relationship in adiabatic process. Work done in any adiabatic (Q=0) process is path independent. We will derive an expression for the potential temperature of an air parcel in terms of its pressure p, temperature T, and the standard pressure p0. Pressure is not inversely proportional to volume for an adiabatic compression.
If the gas is allowed to expand quasi-statically under these so called adiabatic conditions then it does work on its environment, and, hence, its internal energy is reduced, and its temperature changes. Since we have assumed an adiabatic process, –ΔT / Δz defines γ d, the dry adiabatic process lapse rate, a constant equal to 0.0098 K/m, is nearly 1 K/100 m or 5.4°F/1000 ft. "The ideal gas law ( Boyle's Law ) holds that for a confined gas in equilibrium at contact Temperature $T$, that $PV$ is constant, where $P$ is the pressure and $V$ is the volume of the confinement.".
Moles (n) Boyle’s Law (PV = constant). Relationship between Pressure (P), Volume (V), Temperature (T) and quantity;. These calculations are the basis for determining capacity, driver size, and mechanical design.
PV k = constant Note that k is the ratio of specific heats, C p /C v and also sometimes called "γ". In an adiabatic process, the relationship between pressure and volume is PV γ = constant, where γ is the adiabatic exponent that depends on the properties of the gas. Assumptions in Thermodynamic Cycles.
Put Eqn 16 into differential form:. Earth Adiabatic PVT Model Jun19. They are related by a power law that depends on the adiabatic index.
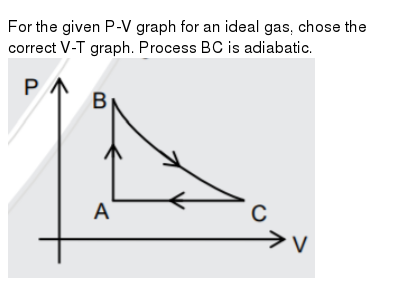
Let us work out the relationship between the pressure and volume of the. Two different adiabatic processes AD and BC intersect two isothermal processes AB at temperature T 1 and DC at temperature T 2 as shown in Figure P21. This is usually called the isothermal gas law.
Adiabatic lapse rate to distinguish it from a process in which condensation or evaporation of water droplets is occurring (the moist or saturated adiabatic lapse rate). The ratio of the specific heats γ = C P /C V is a factor in determining the speed of sound in a gas and other adiabatic processes as well as this application to heat engines. Suppose, now, that the gas is thermally isolated from its surroundings.
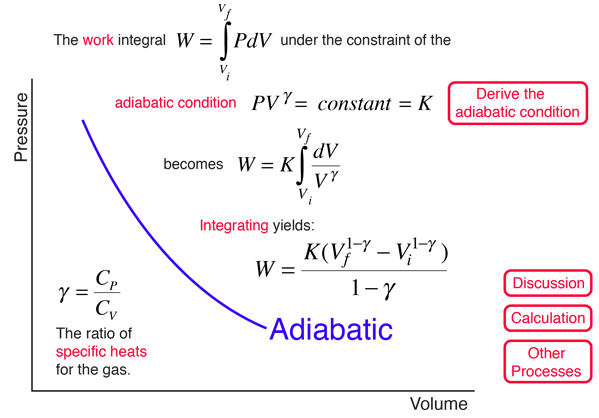
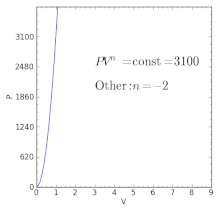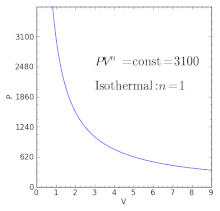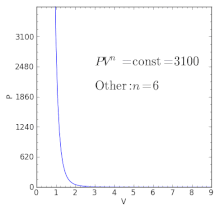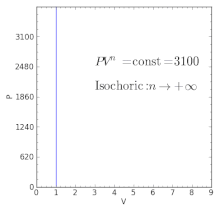
For an ideal gas:. When , the process is isobaric When , the process is isothermal When , the process is isentropic When , the process is isochoric Reversible. Total Work done of the gas is given by:.
This ratio γ = 1.66 for an ideal monoatomic gas and γ = 1.4 for air, which is predominantly a diatomic gas. Based on the adiabatic cooling principle of reducing heat through a modification in close air temperature, advanced adiabatic cooling technologies utilize freely obtainable natural resources, water and clean air, to manage temperature. Isothermal process is a process that happens under constant temperature, but other parameters regarding the system can be changed accordingly.
Initial state of Gas. (a) The upper curve is an isothermal process (ΔT = 0), whereas the lower curve is an adiabatic process (Q = 0). PVT Relationships for Isentropic, IG Processes 8 pts;.
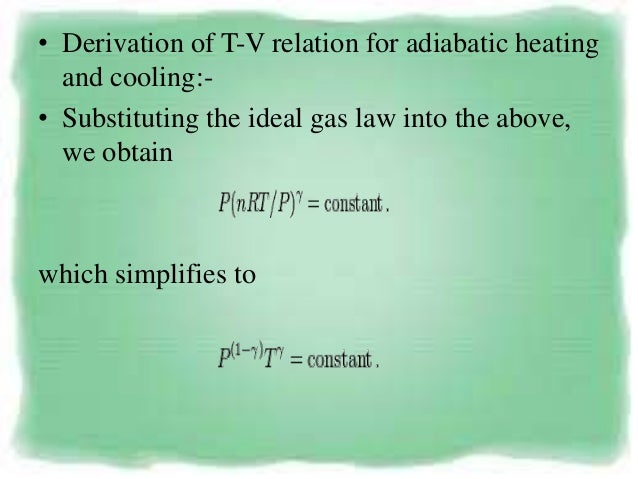
The adiabatic condition of can be written in terms of other pairs of thermodynamic variables by combining it with the ideal gas law. Final state of Gas. Note the relationship between Q 12 and W 12 determined from an energy balance during step 1-2.
Whereas in isothermal process, the temperature remains constant throughout the work. So the equation would become:. Also, we know that P 1 V 1 and P 2 V 2 are equal to nRT 1 and nRT 2 respectively.
First we will apply the 1st Law to adiabatic process 2-3 with no changes in kinetic or potential energy. Hybrid Adiabatic Cooling Tower Process. For a well-insulated closed system, there is usually no heat that flows into the system or leaves the system.
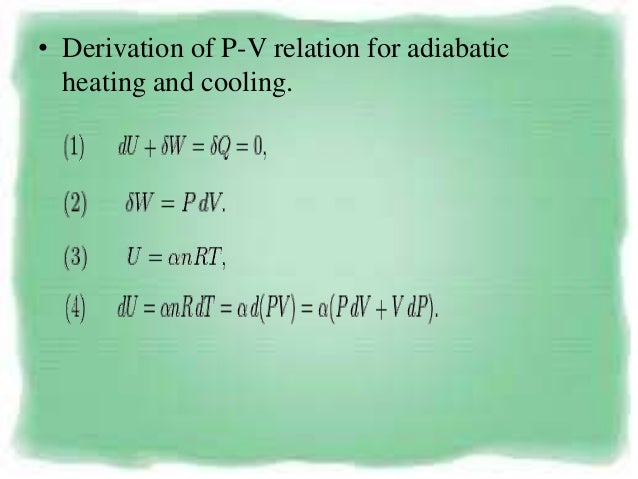
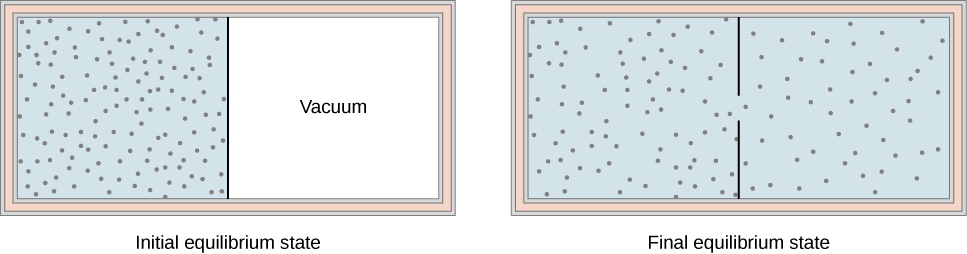
DQ = dU + dW….(1) For one mole of gas, the equation is:. Note the relationship between Q 12 and W 12 determined from an energy balance during step 1-2. Figure \(\PageIndex{1}\) shows a gas confined by a membrane to one side of a two-compartment, thermally insulated container.
An adiabatic process is a thermodynamic process, in which there is no heat transfer into or out of the system (Q = 0). An ideal gas undergoes an adiabatic process obeying the relation PV4/3= constant. The key difference between adiabatic and polytropic processes is that in adiabatic processes no heat transfer occurs whereas in polytropic processes heat transfer occurs.
The polytropic process is a modification of the adiabatic process, involving an efficiency to more closely represent actual conditions. For example, if an ideal gas makes a quasi-static adiabatic transition from a state with pressure and volume and to a state with and then it must be true that. Understand the difference between isothermal and adiabatic process.
Activity cooling systems square measure built to produce customers. Thermodynamics uses the concepts isothermal process and adiabatic process to explain the behavior of a thermodynamic system and its relation to the temperature changes. The two processes used to calculate thermodynamic relationships are isentropic (adiabatic) and polytropic.
The work was also 0 for an isometric process. In general, the process may be too complicated to do the integral (particularly if irreversible process):. The system can be considered to be perfectly insulated.In an adiabatic process, energy is transferred only as work.
Processes, Adiabatic Process, PVT Relationship, PV diagram, TS diagram, Change in Internal Energy, Change in Entropy, Work done, Heat Transferred, Constant Temperature Process, PVT Relationship, PV diagram, TS diagram, Change in Internal Energy, Change in Entropy, Work. Précis We describe the use of a climate model based on the adiabatic meteorological process of daytime lit hemisphere solar radiant forced convection. Thus air cools as it rises and warms as it descends.
Where T is the temperature, CV is the heat capacity at constant volume, and Π is the internal pressure, which is equal to zero for an ideal gas. For an adiabatic process, the polytropic exponent, n, becomes the ratio of specific heats for the gas. Increase in the volume of gas.
Adiabatic processes can occur in well-isolated closed systems. The shaded areas (one positive, one negative) are considered to be proportional to the heat leak. Adiabatic processes can also occur in closed systems that are not.
With everything tied together by the ideal gas law, one variable can always be described as dependent on the other two. Put Eqn 16 into differential form:. THERMODYNAMICS (MOSTLY CHAPTER 19) 198 For an ideal gas the equation of state is pV = nRT (13.50) and dU = nC V dT (13.51) which implies nC.
PV= RT(2) (n =1). A reversible process is one which is performed as if it were always at equilibrium, and without the production on entropy. Well, maybe it's only two variables.
( 1 ) k = Cp / Cv ( 2 ) Ideal gas ( 3 ) Constant specific heats, Cp and Cv ( 4 ) Internally reversible process ( 5 ) Adiabatic process ( 6 ) Delta S = 0.0 ( Isentropic proc. P 2, V 2, T 2:. Adiabatic relationship between P and V.
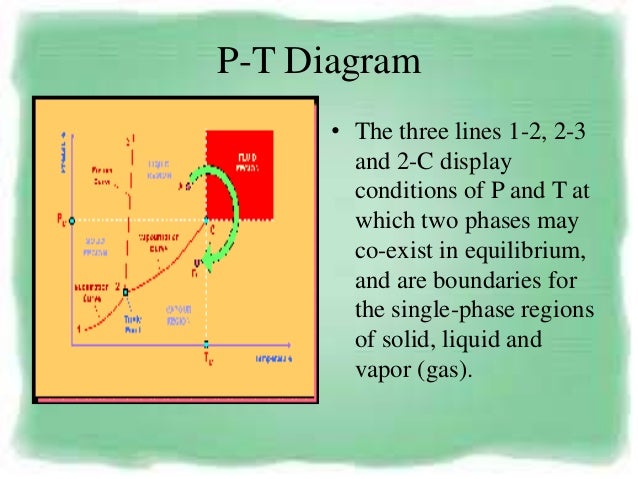
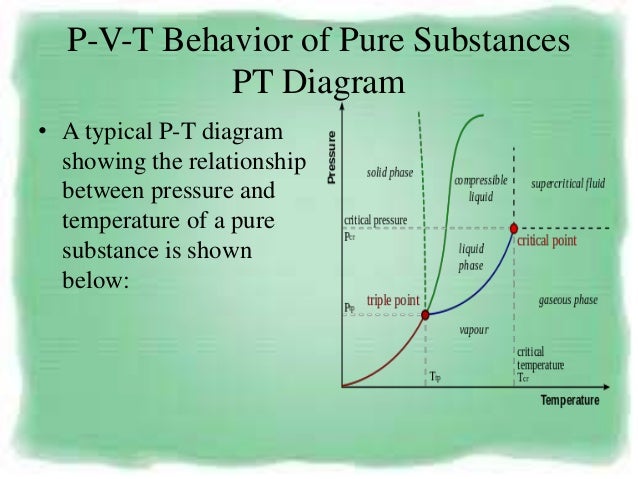
The assumption of no heat transfer is very important, since we can use the adiabatic approximation only in very rapid processes. ( P )( V )^k = Constant is valid for the following conditions:. P-V-T Behavior of Pure Substances PT Diagram • A typical P-T diagram showing the relationship between pressure and temperature of a pure substance is shown below:.
In this video derive an expression for PVT relation of adiabatic process or isentropic process. Making these areas equal is assumed to nullify the heat leak, making the observed temperature change the same as would be observed in an adiabatic process. The process is considered to take place instantaneously along the vertical line.
The mathematical equation for an ideal gas undergoing a reversible (i.e., no entropy generation) adiabatic process can be represented by the polytropic process equation P V γ = constant , {\displaystyle PV^{\gamma }={\text{constant}},}. Equations of state are useful in describing the properties of fluids, mixtures of fluids, solids, and the interior of stars. In the adiabatic process, no heat is added to the system or leaves the system (Q = 0).
Both start from the same point A, but the isothermal process does more work than the adiabatic because heat transfer into the gas takes place to keep its temperature constant. First we will apply the 1st Law to adiabatic process 2-3 with no changes in kinetic or potential energy. DS = n CV ln(Tf/Ti) + n R ln(Vf/Vi) This only depends on the initial state (Vi,Ti) and final state (Vf,Tf), but.
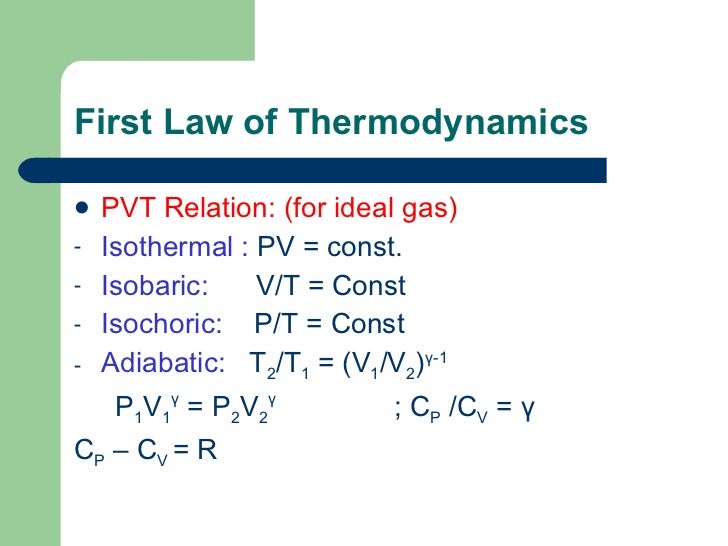
In chemistry, we divide the universe into two parts. A system can be described by three thermodynamic variables — pressure, volume, and temperature. Cp − cV = R.
During an adiabatic process no heat is transferred to the gas, but the temperature, pressure, and volume of the gas change as shown by the dashed line. According to the first law of thermodynamics :. As described on the work slide, the area under a process curve on a p-V diagram is equal to the work performed by a gas during the process.
Where n is the amount of the gas and R is the universal gas constant, and. PVT Relationships for Isentropic, IG Processes. This equation is the condition that must be obeyed by an ideal gas in a quasi-static adiabatic process.
To understand the difference of adiabatic process and isothermal process, one can start from the definition of Carnot Heat Engine.In this article, ACTTR Technology brought to you the relate topics and gave you some ideas of the principles of adiabatic process and. The part we are going to study is “a system”, and the rest is “the surrounding”. Other relations that hold for ideal gases are the ideal gas law.
And then there's the adiabatic process where no heat is allowed to flow into or out of the system. For an adiabatic transformation (dq = 0) the thermodynamic equation is cpdT −αdp = 0 Using the gas equation pα = RT yields cpdT − RT p dp = 0 or dT T = R cp. Adiabatic Process Proof PV^Gamma is Constant, this tutorial is a part of Thermodynamics Tutorial and adiabatic process is really important to find out work d.
If its initial temperature is 300 K and then its pressure is increased upto four times its initial value, then what is the final temperature?. There's the isometric process, also known as isochoric or isovolumetric, where the change in volume is 0, which meant, remember, that means no work can be done. PVT behaviour of gases and relations.
Example applications of the First Law to motivate the use of a property called "enthalpy" (VW, S & B:. This is a special case of the polytropic process since the exponent n, can be any value. Main Difference – Isothermal vs Adiabatic Process.
Describe relationship between heat transfer and adiabatic process An adiabatic process is one in which no heat transfer takes place between a parcel and the environment You get a change in temperature in the air parcel without a transfer of heat energy!. DU = nCvdT, and Cv = dU/dT => dU = nCvdT = CvdT (as n=1) Also, for an adiabatic process, dQ = 0;. Another interesting adiabatic process is the free expansion of a gas.
So the equation for work done in the adiabatic process is also given by. The person who discovered the relationship between volume and pressure of perfect gases under the condition of constant temperature was Select one:. The adiabatic process can be derived from the first law of thermodynamics relating to the change in internal energy dU to the work dW done by the system and the heat dQ added to it.
Deriving an Adiabatic equation. • the gas undergoes an isentropic process → reversible + adiabatic Combining this result with the ideal gas equation of state T 2 T 1 = v 1 v 2 k−1 = P 2 P 1 (k−1)/k The isentropic process is a special case of a more general process known as a polytropic process where → Pvn = constant and n is any number. Relation between PVT Gas Laws:.
From this relationship we can arrive at relationships for several other types of thermodynamic process:. As per thermodynamic terminology, in adiabatic process, there is no exchange of heat from system to its surroundings neither during expansion nor during compression. If an ascending air parcel reaches saturation, the addition of latent heat from condensing moisture will partially overcome the cooling due to expansion.
The turbine is an example of the adiabatic process as it uses the heat a source to produce work. Special Cases n =1 Pv= RT. When the membrane is punctured, gas rushes into the empty side of the container, thereby expanding freely.
Adiabatic process Polytropic process Constant Volume Process Throttling Process. In non isolated systems where there is no adiabatic process, PV is constant. Is the ratio V A /V D greater than, less than, or equal to the ratio V B.
For a cyclic process heat and work transfers are numerically equal;. We can determine the magnitude of the dry adiabatic lapse rate by combing the hydrostatic equation and the first law of thermodynamics for an adiabatic system. In physics and thermodynamics, an equation of state is a thermodynamic equation relating state variables which describe the state of matter under a given set of physical conditions, such as pressure, volume, temperature (PVT), or internal energy.
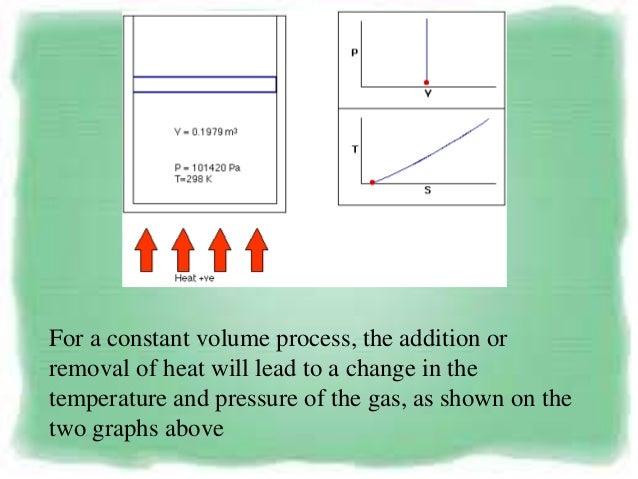
13.8 Adiabatic Process for an ideal gas During adiabatic process dQ =0 ⇒ dU = −dW = −pdV. Constant volume process. Adiabatic Process and Isothermal Process are common terms of thermodynamic while discussing the energy variation in form of heat.
According to the ideal gas equation:.
Pvt Behaviour Of Gases And Relations
Pvt Behaviour Of Gases And Relations
Process Calculation In Ideal Gas
Pvt Behaviour Of Gases And Relations

Physical Chemistry I Tkk 2246 13 14 Semester 2 Instructor Rama Oktavian Office Hr M 13 15 Tu W Th Ppt Download
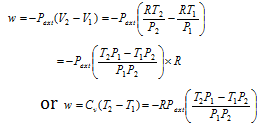
Adiabatic Expansion Of An Ideal Gas Reversible Irreversible Adiabatic Expansion Thermodynamics Chemistry
Http Pu Edu Pk Images Image Lecture Notes Advanced Chemical Engineering Thermodynamics Pdf

Pvt Behaviour Of Gases And Relations

Example 7e 4 Performance Of An Ideal Gas Cycle

Adiabatic Process An Overview Sciencedirect Topics

Pvt Behaviour Of Gases And Relations

Thermodynamic Properties Property Table W Property Table From Direct Measurement W Equation Of State W Equation Of State Any Equations That Relates Ppt Download

Search Youtube Channels Noxinfluencer

Prove The Adiabatic Relation Youtube

Search Youtube Channels Noxinfluencer

What It The Temperature And Volume Change After Air Is Compressed From 1 Ksi To 10 Ksi Thermodynamics

Derivation Pv Gamma Youtube
Pvt Behaviour Of Gases And Relations
Derive The Relationship Between Pressure And Volume In Adiabatic Change Quora
Pvt Behaviour Of Gases And Relations

260h Licensed For Non Commercial Use Only Expansion And Compression Work 13

A Monatomic Ideal Gas Initially At 17oc Is Suddenly Compressed To 18 Of Its Original Volume The Temperature After Compression Is

Relation Between P V T For An Ideal Gas Undergoing Adiabatic Chan

Search Youtube Channels Noxinfluencer

Adiabatic Process Isentropic Process P V T Relation Thermodynamics Youtube

Adiabatic Process Relation Between P V And T Testbook

What Is The Relation Between Temperature Pressure And Volume In An Adiabatic Process Thermodynami Youtube

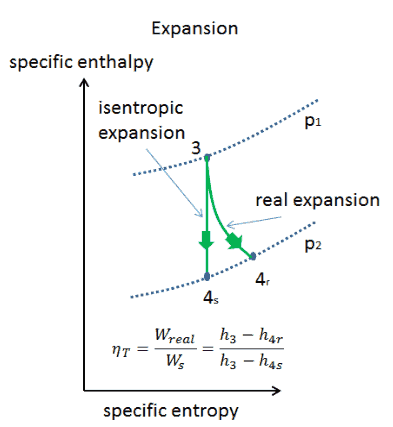
Thermodynamic Relationship An Overview Sciencedirect Topics

What It The Temperature And Volume Change After Air Is Compressed From 1 Ksi To 10 Ksi Thermodynamics
2
Pvt Behaviour Of Gases And Relations

Chapter7 Lesson E Pvt Relationships For Isentropic Ig Processes

Diesel Cycle Diesel Engine

Adiabatic Process Relation Between P V And T Testbook
Relation Between P V T For An Ideal Gas Undergoing Adiabatic Chan
Q Tbn 3aand9gcspcligjfybcmci22esegzqz5rhiu7mjz4lhf2zzvo O Guoa4n Usqp Cau

Adiabatic Process Relation Between P V And T Testbook

Adiabatic Process P V T Relation Youtube

Are You Supposed To Use The Internal Or External Pressure For The Pv Work Integral Physics Stack Exchange

Adiabatic Process Relation Between P V And T Testbook

Chapter 2 Simple Thermodynamics Systems Ppt Video Online Download
Adiabatic Processes

Adiabatic Process Relation Between P V And T Testbook

Thermo Chapter 4 Lecture Pdf Gases Enthalpy
Revision On Thermodynamics

Chapter 3d The First Law Closed Systems Otto Cycle Engines Updated 4 22 12

Relation Between P V T For An Ideal Gas Undergoing Adiabatic Chan
3 7 Adiabatic Processes For An Ideal Gas Physics Libretexts

271f10l12 Physics Labs

Thermodynamic Relationship An Overview Sciencedirect Topics
What Is Adiabatic Process Definition
2

Thermodynamic Properties Property Table W Property Table From Direct Measurement W Equation Of State W Equation Of State Any Equations That Relates Ppt Download

Polytropic Process As A General Process Thermodynamics Youtube

Proof Of Pressure Volume And Temperature Ratio Adiabatic Process Youtube

Isobaric Process Wikipedia
2

What It The Temperature And Volume Change After Air Is Compressed From 1 Ksi To 10 Ksi Thermodynamics

Adiabatic Process P V T Relation Youtube
Pvt Behaviour Of Gases And Relations
011 Second Law Cycle Analysis
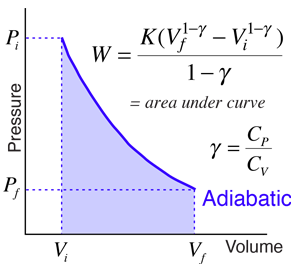
Adiabatic Processes
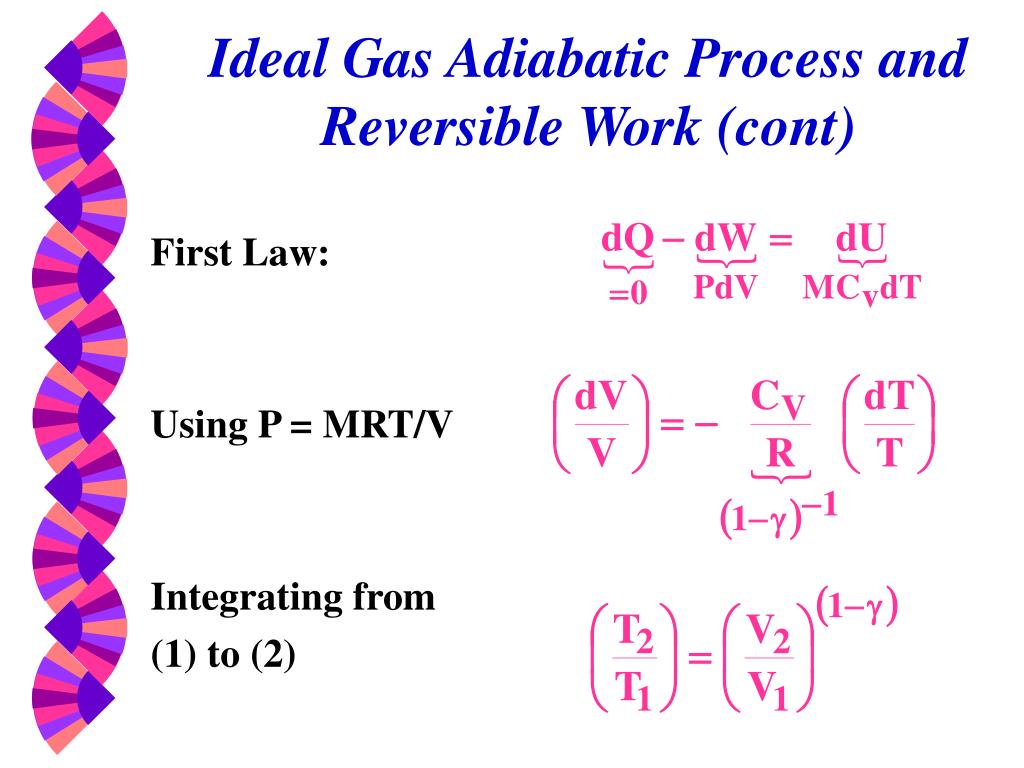
Ppt Thermodynamic Properties Powerpoint Presentation Free Download Id 5036
2
Q Tbn 3aand9gcsmfurz9sqcorawss2udjngowaf17gbnno7p6jwkjhtquv5jnvf Usqp Cau

Adiabatic Process Study Material For Iit Jee Main And Advanced Neet Aipmt Askiitians
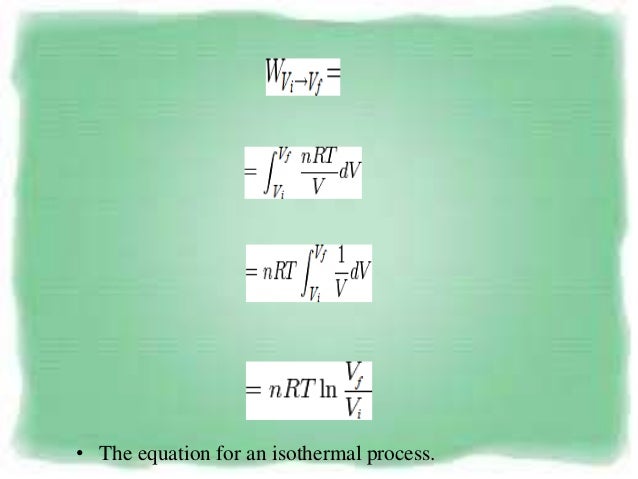
Pvt Behaviour Of Gases And Relations
Derive The Relationship Between Pressure And Volume In Adiabatic Change Quora

The Relation Between The Temperature And Volume In Adiabatic Process Download Scientific Diagram

Relation Between P V T For An Ideal Gas Undergoing Adiabatic Chan

Chapter 3d The First Law Closed Systems Otto Cycle Engines Updated 4 22 12

Polytropic Processes For An Ideal Gas Youtube

Adiabatic Process Wikipedia

Thermodynamics I Unit I 2nd Semester Suggested Books Ppt Download
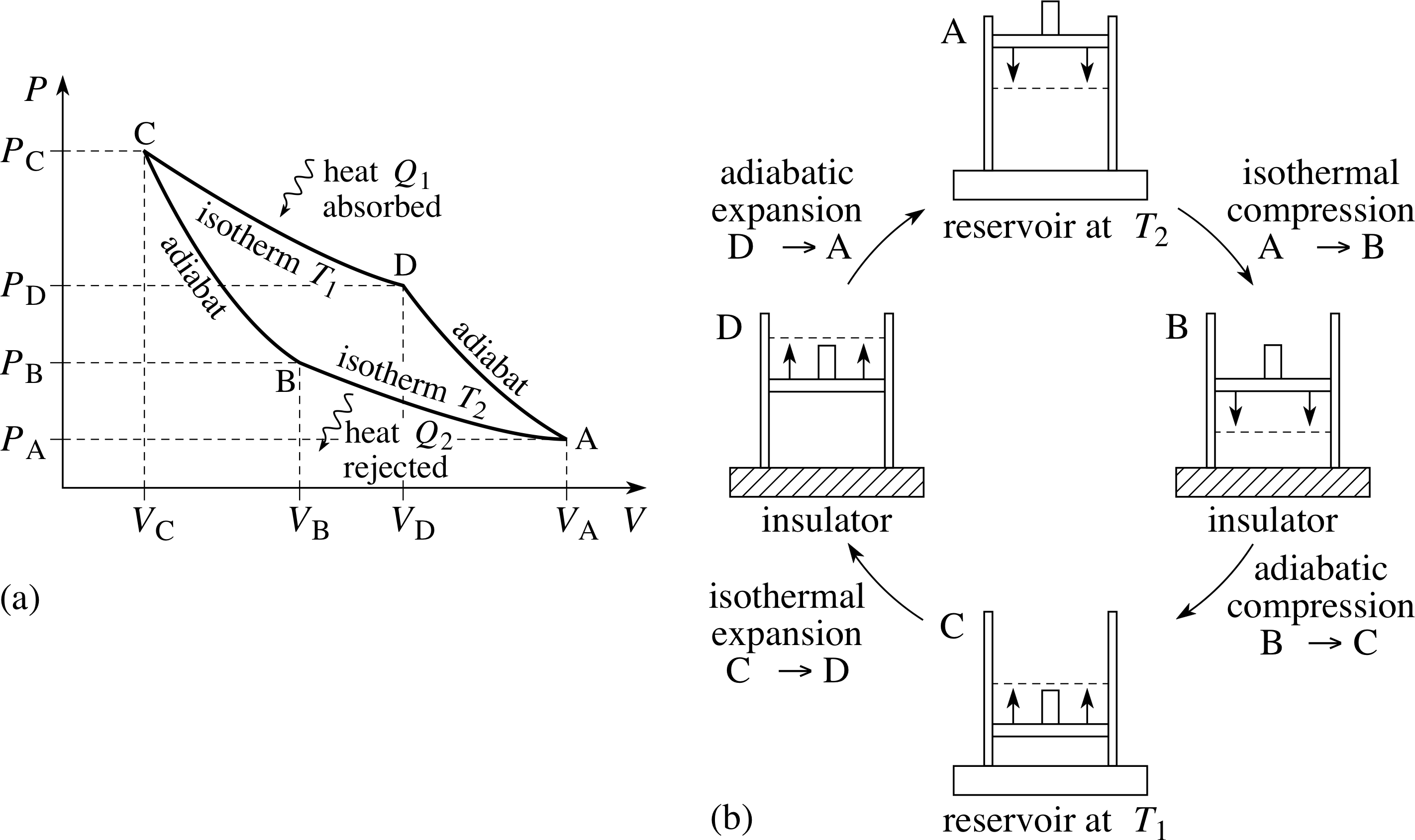
Pplato Flap Phys 7 4 Specific Heat Latent Heat And Entropy

Chapter 3c The First Law Closed Systems Diesel Cycle Engines Updated 3 19 13
Q Tbn 3aand9gcqxbypmefsvwvlwh Alivwm3bswl5fqpkrnkw Usqp Cau

Relation Between P V T For An Ideal Gas Undergoing Adiabatic Chan
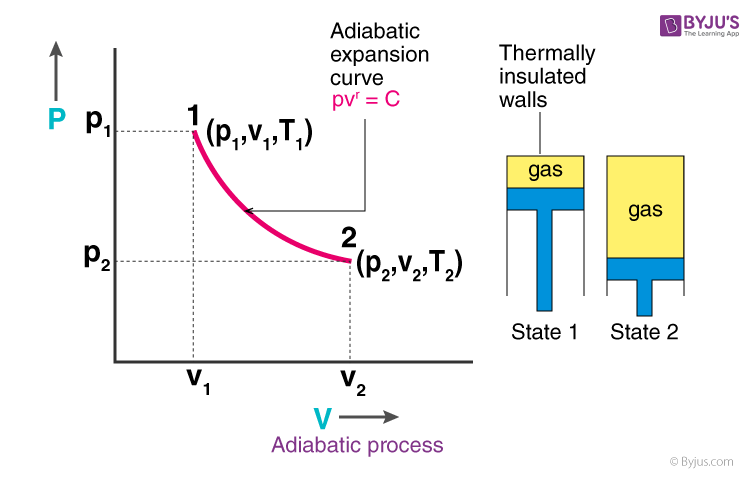
What Is Adiabatic Process Equation Reversible Diagram Example

Derivation Of The Relation Between Temperature And Pressure For An Irreversible Adiabatic Expansion Chemistry Stack Exchange
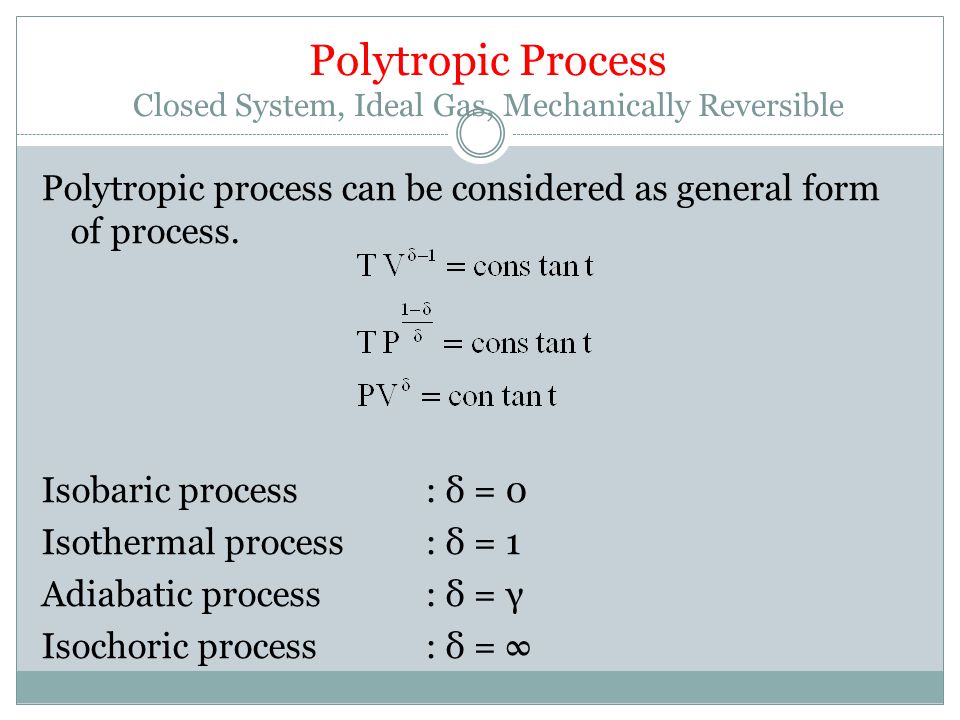
Itk 233 Termodinamika Teknik Kimia I Ppt Video Online Download
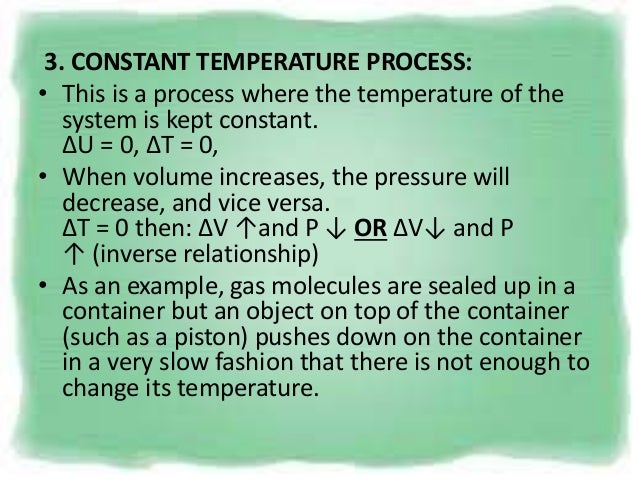
Pvt Behaviour Of Gases And Relations
Q Tbn 3aand9gctjr9fbxgbybpkabbehuartnrctazxhjjo Uf1 Mx48yks1zppc Usqp Cau
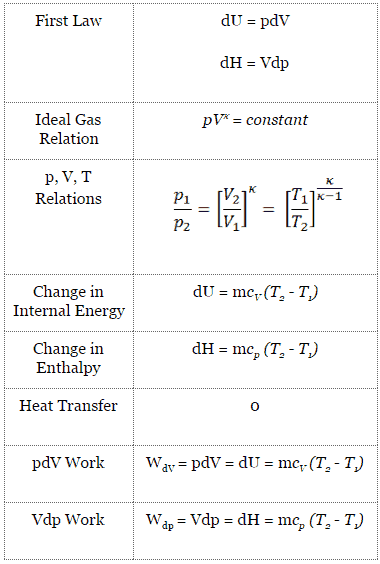
What Is Adiabatic Process Definition
Q Tbn 3aand9gctuzl Y7clznasql1tb1ip Ip6j3sf6vforjz1 Nte9obzfi8sq Usqp Cau

Ch18 Ssm
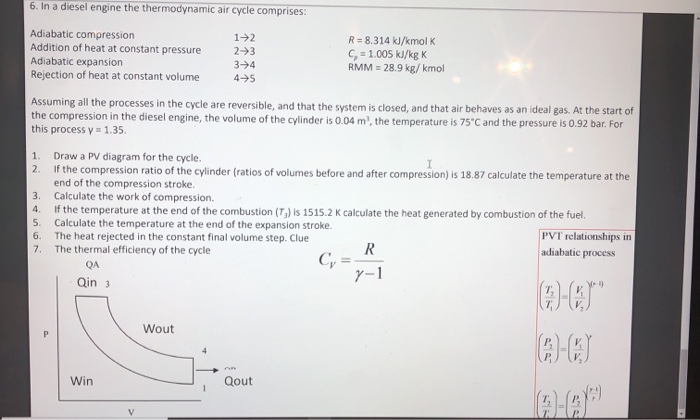
Solved 6 In A Diesel Engine The Thermodynamic Air Cycle Chegg Com

Are You Supposed To Use The Internal Or External Pressure For The Pv Work Integral Physics Stack Exchange
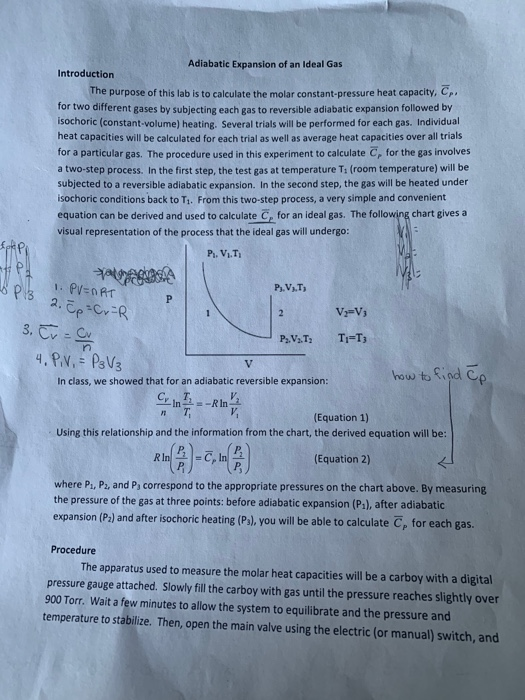
Solved Derive Equation 2 From Equation 1 Other Known Equt Chegg Com

Isentropic Process Nuclear Power
Http Emreyalamac Cbu Edu Tr Wp Content Uploads 17 11 Mse2105 Lecture3 Pdf
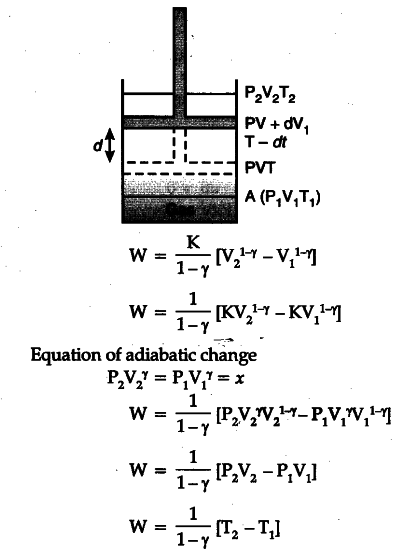
Derive An Expression For Work Done In Adiabatic Expansion Cbse Class 11 Physics Learn Cbse Forum
2

13 Thermodynamics Proof Of Adiabatic Equation Most Important Complete Concept Youtube

Ch Thermodynamics By Career Avenues Issuu

Pptx Thermodynamic Applied And Interdisciplinary Physics Continuum Mechanics
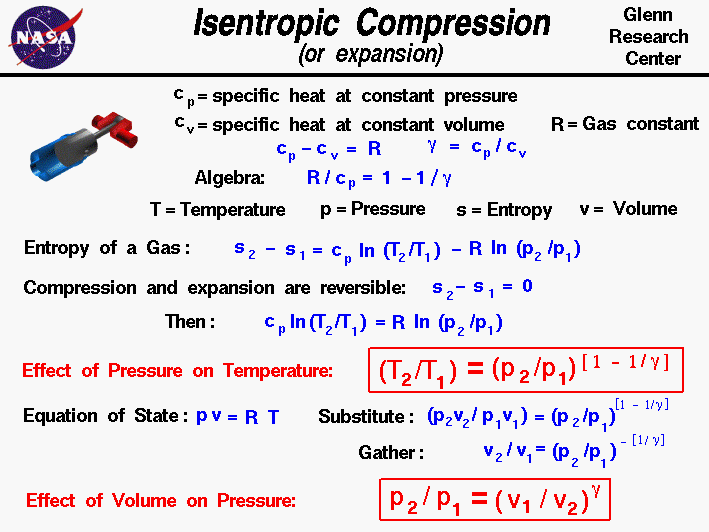
Isentropic Compression Or Expansion

What Is The Relation Between Temperature Pressure And Volume In An Adiabatic Process Thermodynami Youtube



